Iron (Fe) contamination was first recognized as a concern for fluid catalytic cracking (FCC) units in the 1990s. BASF has studied iron poisoning and its effects on FCC catalyst extensively, and a short summary of some of those findings is included here.
First, it is important to understand iron sources and how to quantify iron on FCC catalyst. Iron is inherent in the fresh catalyst, between 0.25 and 0.75 wt%, as it is a natural ingredient of clays used for manufacturing FCC catalysts; this type of iron does not participate in any side reactions nor cause any kind of catalyst surface blockage. The iron that is of concern comes in with the crude oil in the form of iron incorporated in high molecular weight hydrocarbons. Iron can also come from equipment corrosion (tramp iron), which is generally less of a concern. Iron from the feed deposits on the surface of the FCC catalyst, forming nodules. This is referred to as added iron.
Concerns with iron-poisoned catalysts
Units with iron-poisoned catalysts can face a number of problems, either from the chemical or physical effects of added iron. The chemical effects are often minor and include higher hydrogen (H2) and coke due to the dehydrogenation activity of iron, mild carbon monoxide (CO) promotion, and the transfer of sulfur (S) from the reactor to the regenerator, which can increase sulfur oxide (SOx) emissions. The physical effects include nodule formation (impacting catalyst circulation), vitrification and potentially reduced surface area. Severe poisoning leading to surface blockage, in which the pores are not accessible, is the most common concern. Surface blockage can prevent feed molecules from diffusing into the catalyst in the riser and products from diffusing out, leading to lower conversion, higher coke and higher slurry.
The amount of added iron that causes significant surface blockage and results in a loss of conversion depends heavily on the type of catalyst, the source of iron and other contaminants. Catalysts with optimized porosity, especially surface porosity, give improved iron tolerance. BASF practices in-situ manufacturing, which has been shown to improve iron tolerance.1 Based on surface morphology, in-situ manufactured catalysts have high surface porosity, which can withstand a higher degree of fouling due to iron. BASF catalysts have been successfully used in commercial applications with above 2 wt% equilibrium catalyst (Ecat) iron.
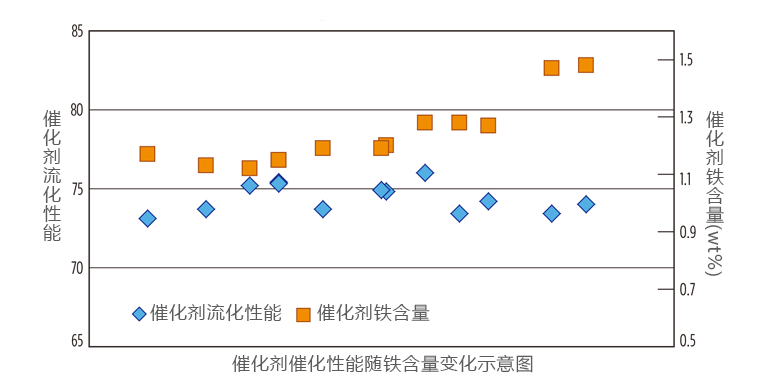
No loss of conversion
BASF follows iron upsets closely in the industry. In the past 15 years, BASF catalysts have not experienced a loss of conversion due to an iron upset. The case study below describes an iron excursion at a North American (NA) refinery using BASF’s Stamina catalyst. Over the course of a few months, the refinery introduced high iron feed into the FCC unit. The refinery saw iron on Ecat increase from 1.17 wt% to 1.48 wt%, representing a 45% increase in added iron (iron coming from the feed), as shown in TABLE 1. At the same time, they also saw calcium (Ca) and sodium (Na) on Ecat increase 23% and 48%, respectively, while nickel (Ni) and vanadium (V) werestable. The unit experienced no loss in activity or higher slurry yields (FIG. 1). Of note, when an increase in iron accompanies a loss in conversion, the higher iron is often an indicator of a feed change and does not cause the loss of conversion. In these cases, it is the heavier feed and increases in Na and V that result in the decreased conversion.
Differentiating iron mobility.
Another area of BASF’s iron research is in the mobility of iron. When looking at iron mobility, or any metal mobility for that matter, it is important to differentiate between intraparticle mobility (within a single catalyst particle), and interparticle mobility (from particle to particle). One method of quantifying intraparticle mobility is by using scanning electron microscopy (SEM) data of cross sectional areas of Ecat particles. Comparing the amount of contaminant metal on the outer stages vs. the inner stages gives the peripheral deposition index (PDI).2 For context, vanadium typically has PDI values of close to 1, indicating close to uniform content of vanadium throughout the particle and thus high intraparticle mobility. Measured iron PDI values range from 4 to over 7, indicating low intraparticle mobility, similar to the deposition profile of nickel.
For interparticle mobility, a variety of techniques have been used to study bulk Ecat. Metals analysis of sink-float separated catalyst particles, where fractions are separated based on density, shows a standard deviation among fractions for iron between those of vanadium (high mobility) and nickel (low mobility). Looking at bulk Ecat in SEM, it can clearly be seen that there is a distinction between new (no iron) and old (high iron) catalyst particles via nodulation and surface mapping, indicating that the mobility profile of iron is distinctly different from that of V. In summary, across
a number of commercial units studied, iron does not show high interparticle mobility
like V.
Stamina: Fluid Catalytic Cracking (FCC) catalyst
Stamina is a distillate maximization Fluid Catalytic Cracking (FCC) catalyst providing measurable improvements in bottoms upgrading, light cycle oil quality and output, while preserving low coke yields.
Stamina is a Fluid Catalytic Cracking (FCC) catalyst for maximizing distillate yield from resid feeds
Stamina is a premium FCC catalyst that helps refiners process resid feedstocks to meet the growing global demand for diesel fuels.
How does Stamina work?
Stamina? is based on the new Prox-SMZ Technology Platform and combines the high matrix activity needed for light cycle oil (LCO) maximization and the enhanced macroporosity required for resid molecule access. The result — a high activity, high-stability matrix technology that cracks the heaviest resid feeds to maximize diesel yields.
What is the Prox-SMZ Technology Platform?
BASF has developed an innovative catalytic technology platform for maximizing distillate yields from a fluid catalytic cracking (FCC) unit. This unique platform, designated Prox-SMZ for Proximal Stable Matrix and Zeolite, combines attributes from both high zeolite and high matrix activity catalysts. The product is a stable and selective catalyst specifically designed for FCC distillate maximization.
What can Stamina do for refiners?
Stamina? can help refiners meet the increased global demand for diesel fuels from the severest feeds. Recent trials have successfully demonstrated how well Stamina? can process feeds with higher levels of contaminant metals while reducing slurry yields.

 石油圈
石油圈