The following is a review of challenges associated with seawater injection and their respective mitigation strategies, as well as the geographic distribution of seawater injection projects. The discussed challenges include plugging, due to suspended solids and bacteria; deterioration in well injectivity; the corrosive effect of injection water on the injection system; scaling effects of seawater injection when used in conjunction with produced water re-injection; and reservoir souring. The mitigation strategies discussed include types of filtration systems, in-situ chlorination, chemical addition, de-aeration systems, and sulphate removal systems. Finally, suggested industry best practices for seawater injection systems are presented in this article.
INJECTION CHALLENGES
Seawater injection is done for purposes of maintaining reservoir pressure and also as a form of improved oil recovery, by sweeping oil from the injector wells to the producing wells. These two purposes of seawater injection are required for two reasons. The first requirement, to maintain reservoir pressure, is due to reservoir pressure depletion as a result of production activities. The second requirement, to improve oil recovery, is due to the limited hydrocarbon recovery from primary production.
Seawater injection challenges include:
1.Plugging, due to suspended solids
2.Deterioration in well injectivity
3.Corrosion
4.Scaling
5.Reservoir souring.
Plugging, due to suspended solids. Plugging reduces reservoir porosity, when suspended solids block the pore throat of the reservoir. This reduces well injectivity and increases the required injection pressure. The risk of plugging of the reservoir by suspended solids can be reduced by applying filters.
Deterioration in well injectivity. The deterioration in well injectivity can be characterized by the skin effect. The skin effect is a measurement of the damage in the near-wellbore region. Near-wellbore region damage could result from scaling, hydrocarbon phase changes, or other production activities that reduce the reservoir’s permeability. Deterioration in injectivity can be remediated by well workovers for well stimulation purposes.
Corrosion is an electrochemical reduction and oxidation process. It involves anodic and cathodic reactions occurring on the metal. Corrosion leads to metal depletion, metal wastage, and metallic embrittlement and cracking, which can lead to equipment failure. Corrosion is an irreversible chemical process. The treatment for corrosion involves applying the following corrosion prevention methods:
1.The use of corrosion-resistant alloys (CRAs);
2.The application of chemicals, such as corrosion inhibitors, biocides and nitrate injection;
3.The application of de-aeration (mechanical and chemical) to prevent oxygen corrosion.
Corrosion-resistant alloys. The use of corrosion-resistant alloys involves the application of materials made from alloys that offer some level of resistance to corrosion.
Corrosion inhibitors. The application of chemical corrosion inhibitors is used to prevent the formation of the electrolytic cell required for corrosion to occur. Corrosion inhibitors can be classified as either organic or inorganic. Inorganic corrosion inhibitors can be passivating (i.e. anodic), or they can be cathodic. Passivating corrosion inhibitors work by forming a thin film of non-reactive surface on the metal. Cathodic corrosion inhibitors inhibit the reduction of water to hydrogen. Organic corrosion inhibitors (also called film-forming corrosion inhibitors) are typically preferred in oil, gas and refining activities. They affect the entire metal surface and generate a protective barrier.
De-aeration. Seawater de-aeration can be achieved by mechanical treatment or by chemical treatment. Mechanical de-aeration involves the utilization of de-aeration towers to reduce dissolved oxygen content in sea water, to levels of less than 20 ppb. Some methods involve the use of gas stripping, vacuum de-aeration, compact de-oxygenation (e.g., MINOX system), and the wet combustion catalytic process. Chemical treatment involves the use of oxygen scavenger chemicals to react with the dissolved oxygen in the water. Examples of oxygen scavengers include sodium sulfite, sodium bisulfite, and ammonium bisulfite.
Scaling. Scale is an inorganic deposit that precipitates from the water phase of produced fluids. Examples of scale include: calcite anhydrite (CaCO3), barite (BaSO4), halite (NaCl), iron sulfide (FeS), calcium sulfate (CaSO4) and siderite (FeCO3).
Scale inhibition strategies include:
1.The application of chemical scale inhibitors
2.The removal of scale-forming constituents.
Scale inhibitors. Chemical scale treatment involves the use of scale inhibitors to prevent scale formation. The mechanism by which the scale inhibitor works is either nucleation inhibition, crystal growth retardation, dispersion or chelation. The chemical can be applied by continuous treatment, batch treatment or squeeze treatment.
Removal of scale-forming constituents. The removal of scale-forming constituents can involve the application of a sulphate removal package (SRP). This is a membrane separation process, typically applied to avoid the formation of barium sulfate scales, which can be difficult to remediate. The SRP treatment application can reduce sulfate in sea water to less than 20 ppm. SRP’s can be capital-intensive, of large weight and footprint, and of high maintenance. A SRP uses a nano filtration system that reduces the seawater sulphate ions, from approximately 2,800 mg/l to 100 mg/l or less. This will reduce the potential for the formation of sulphate scale.
Reservoir souring treatment. Reservoir souring involves a reservoir that was initially sweet (i.e., with no H2S production), becoming sour with H2S being produced in the production wells. This typically occurs after a period of injection of sulfate containing water. The H2S is being produced by sulphate-reducing bacteria.
The chemical treatment of the reservoir souring includes the application of aerobic bacteria biocides, anaerobic bacteria biocides, nitrate injection and the utilization of a SRP to reduce the ions available to reducing bacteria (SRB).
SEAWATER INJECTION: BEST PRACTICES
The following are some recommended practices for seawater injection systems:
1.Utilize high-quality feed water by ensuring that caisson water depth for seawater intake is at least 50 m below the sea surface.
2.Avoid oxygen ingress through leaks, in and around pipe connections and process equipment, to prevent oxygen corrosion
3.Perform proper maintenance of vacuum pumps, to maintain de-aeration efficiency
4.Practice a robust monitoring system
5.Use on-line particle measurement for monitoring water injection systems’ solids distribution
6.Regularly monitor process data, such as water lift rate, pressure readings, and water injection rate for irregularities, which can be signs of injection problems.
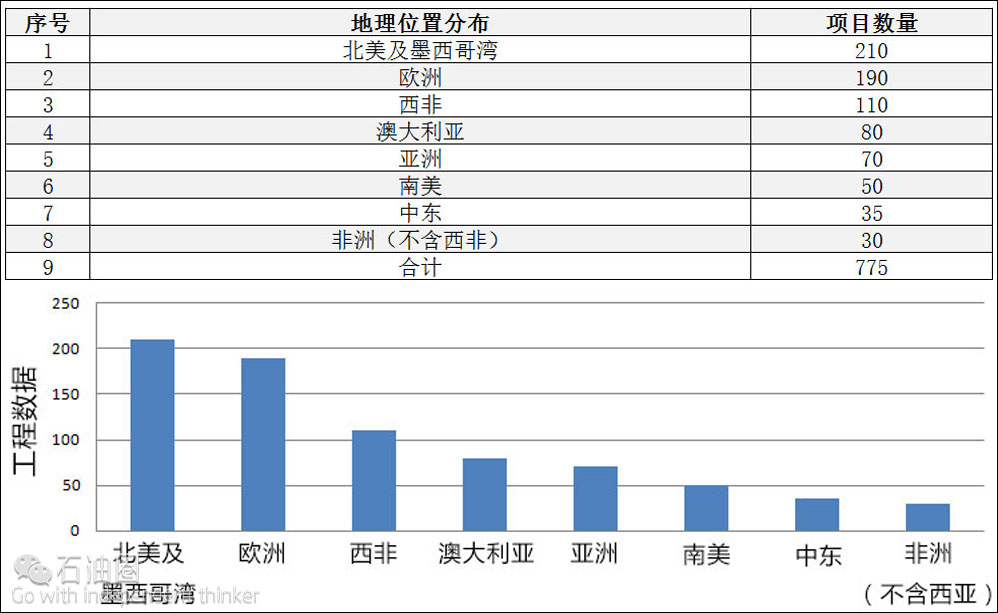
Seawater injection: Geographic distribution. The Table and Fig show the estimated number of global active, or planned, deepwater, seawater injection projects by geographic location. This compilation is based on data available at the time of preparation of this material, and is presented to give an appreciation of the global distribution of seawater injection activity for deepwater projects.
Table. Estimated number of active/planned, deepwater, seawater injection projects, based on available data.
Fig. North America (including the Gulf of Mexico) and Europe, combined, account for more than half of all deepwater, seawater injection projects, either active or planned.


 石油圈
石油圈