Subsurface DNA diagnostics aid well spacing decisions in the Permian
Lessons are learned from more than 10,000 produced fluids and cuttings samples from more than 200 wells.
The oil and gas industry’s newest diagnostic tool, subsurface DNA diagnostics, has recently hit an important milestone: 10,000 subsurface samples from the Permian basin. To date, Biota Technology has sequenced more than 10,000 produced fluids and cuttings samples from more than 200 wells, resulting in 388 million subsurface DNA markers. Data science and machine learning tools have been coupled with this unprecedented dataset to translate the millions of DNA markers into actionable insights for Permian operators.
The integration of these findings has helped to resolve the complex picture of the subsurface by revealing significant out-of-formation contribution from stacked laterals, and well-to-well communication during completions.
Well spacing decisions are paramount for maximizing EUR and net present value in the highly stacked Permian. However, operators face several key challenges in their quest to optimize field design. Efficient lateral spacing is confounded by lateral heterogeneities and stratigraphic changes within the basin. Vertical spacing requires time-lapse drainage height monitoring to ensure adequate calibration. In addition, translating science-well findings into generalizable knowledge requires a great deal of data, both within a wide geographic range, as well as a temporal basis through the lifecycle of a field.
The scalability of DNA sequencing has enabled time-lapse drainage height findings and rapid upscaling from individual wells and pads to field-wide characterization. These advancements are helping operators place increasingly closer laterals and stacked targets into subsurface sections, and monitor drainage volumes through their fields’ lives.
SUBSURFACE DNA DIAGNOSTICS IN THE PERMIAN
The Permian basin is a multi-stacked play, where production wells potentially can draw resources from several different zones, creating a compelling need for accurate contribution estimations on a per-formation, per-well basis. The Bone Spring formation has been a frequently drilled and prolific zone in the Delaware basin, with production rapidly increasing since 2008.1 Recently, drilling campaigns have also targeted the Wolfcamp shale play, the liquids-rich Bone Spring sands, and the Avalon shale play.
Together, these stacked benches represent significant potential for increased efficiency, including multi-well pads, extended laterals, enhanced completion designs, and horizontal well spacing. However, in these relatively thick packages, efficient development is complicated by vertical heterogeneity driven by lithology, significant lateral variability from the basin center to margins, and mechanical stratigraphy changes. Due to these complications, effective stacking of laterals is the key driver in basin economics, and monitoring infill wells to determine relative drainage, and communication between wells, is an ongoing challenge.
DETERMINING VERTICAL DRAINAGE HEIGHT
Seeking to acquire an increased understanding of vertical drainage height in the Delaware basin, Anadarko Petroleum and Biota conducted a field-wide trial of subsurface DNA diagnostics.2 Initially, 174 produced fluid samples were collected from formations A, B and D within the Wolfcamp for the analysis. This number has subsequently increased to include significantly more fluid samples and greatly expanded well coverage, to expand evaluation of field-wide variations and long-term understanding of the operator’s acreage.
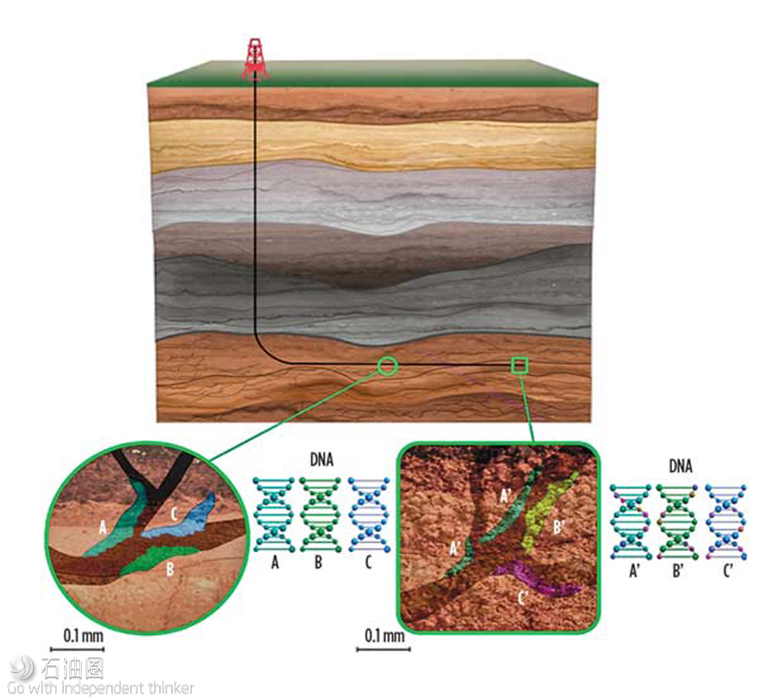
Fig. 1. Subsurface DNA originates from microbes that live in the fracture networks and pore space of rocks. These DNA markers capture stratigraphy and lateral heterogeneity at a higher resolution than current methods applied to the subsurface.
Subsurface DNA markers originate from microbial communities that can survive in elevated temperatures and pressures that are common to reservoir systems.3 Microbes are ubiquitous in subsurface environments, residing in hydrocarbons and formation waters, as well as within rock-associated pore spaces and fracture porosity.4 They consume a wide range of carbon sources (including hydrocarbons) and respire with various redox-elements, creating diverse systematic variation, due to the localized environment, Fig. 1.
High-resolution studies into the subsurface DNA of the Delaware and Midland basins return hundreds of millions of DNA sequences, comprising tens of thousands of unique DNA markers. This staggering diversity is driven by the high selectivity of microbes for subsurface conditions, such as lithology, pressure, and reservoir fluid composition. The confluence of these factors allows subsurface DNA markers to serve as formation-associated fingerprints, influenced by geology and post-diagenetic processes. Microbial communities consume a wide range of carbon sources (including hydrocarbons) and respire with various redox-elements, creating diverse systematic variation, due to localized environment. As reservoir and subsurface conditions change, these variations can be observed through alterations in microbial communities. In fact, resolution at sub-formation level to per-TVD level has been observed in subsurface DNA markers within the Permain basin.
Samples of subsurface DNA taken from produced fluids or well cuttings provide a snapshot of the subsurface returning tens of thousands of DNA markers per sampling point. The DNA sequencing step introduces minimal bias, meaning that end-members do not need to be re-sequenced when new samples or time points are added to the analysis. This enables quick turnaround times and scalability on field-wide profiling over time. The preservation of the end-members in time, and paired comparisons of new and existing wells, are key advantages when monitoring the life and development of an asset.
INTEGRATION OF SUBSURFACE DNA AND DATA SCIENCE
From the initial analysis of Anadarko’s wells, the 174 fluid samples returned thousands of unique subsurface DNA markers. Twenty-six of the wells were landed in distinct sub-formations within the basin, as Formation A accounted for 11 wells and Formation B accounted for 15 wells. The formation assignment was used to build a data science model for the formations. Using the model, the initial screening of the DNA markers associated with these wells indicated strong biological similarity between subsurface DNA markers from the two formations.2
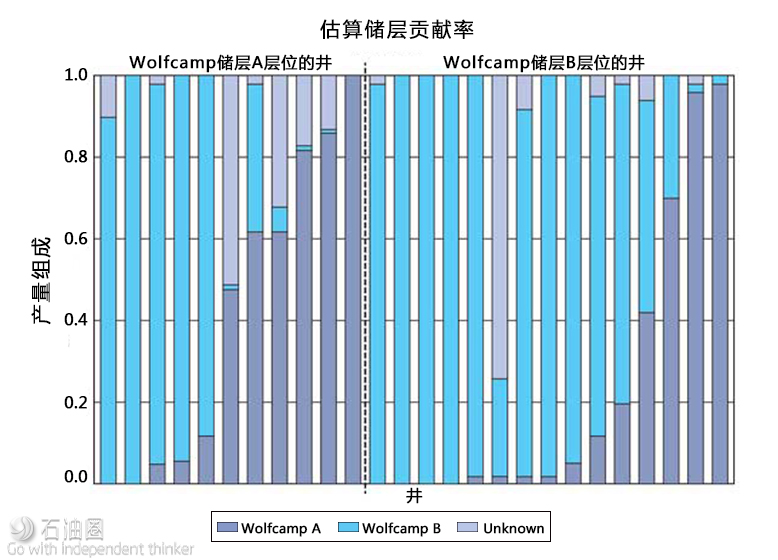
Fig. 2. Estimating the contribution from Formation A and Formation B (both within the Wolfcamp) to each well. Wells to the left and right of the black vertical bar landed in Formation A and Formation B, respectively. The wells are sorted within the formation of landing by the estimated contribution of Formation A.
To determine the relative drainage height for wells landed in these formations, subsurface DNA markers from produced fluids were used to create computational end-members for formations A and B, for the 26 wells. The data science approach of computationally determining the end-members has previously been applied and refined in other unconventional plays, where it has shown significant agreement with other diagnostic techniques, such as micro-seismic operations.
The results of the initial analysis suggest out-of-formation production in both formations, Fig. 2. The findings of commingled flow were the impetus for an expanded project with the operator. Ongoing monitoring will widen the scope of this project, through an increased collection of samples, to determine the spatial and temporal variation for non-formation contribution.
TEMPORAL VARIATION IN PRODUCED FLUIDS
Time-based analysis along the life of a field has the potential to track drainage height at unprecedented resolution during production.
Subsurface DNA markers from produced fluids have been shown to contain sufficient resolution to delineate commingled flow from stacked reservoirs. In a deployment of Biota’s technology in the Northwest shelf with another operator, three stacked formations were explored, to ascertain whether subsurface DNA could determine relative contributions from a commingled flow.
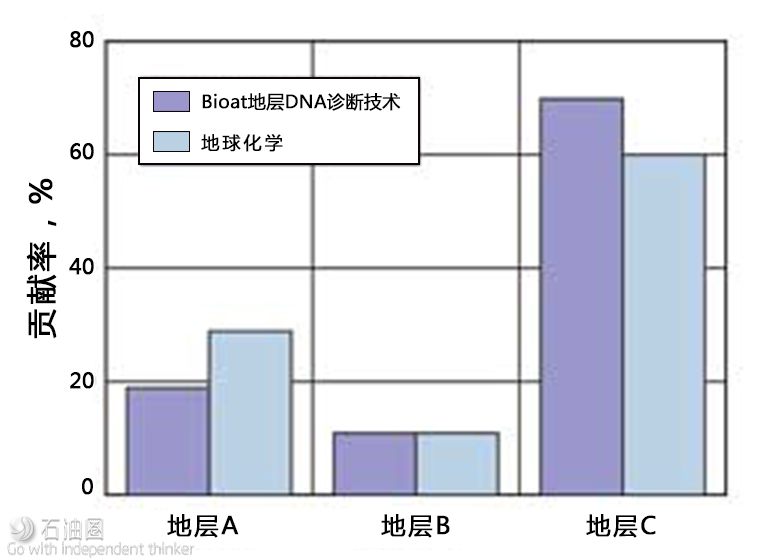
Fig. 3. Formation allocation for a commingled oil sample taken from the three stacked benches in the Northwest shelf. End-members for subsurface DNA and geochemistry were taken from vertical parent wells and used to determine production allocation.
The subsurface DNA end-members were obtained from three separate vertical wells. One landed in each formation, each of which had been online for more than 600 days. Commingled fluids were then sampled from another well to determine production allocation of these three formation endmembers. Biota’s analysis of DNA markers indicated that the majority of fluid contribution came from Formation C, with less contribution from formations A and B, Fig. 3. A paired set of these fluids was sent for geochemical analysis and analyzed independently, per the operator’s standard operating procedure. The DNA findings corroborated with the operator’s geochemical analysis and provided independent validation of the subsurface diagnostics.
In a separate investigation of two wells in the Delaware basin, the temporal variation of DNA markers in fluids from early flowback, following completion, was explored at high resolution. DNA markers over the first month had significant variation, likely due to the commingling of completion fluids with reservoir fluids, with signals stabilizing within the first few months. Monthly monitoring of new wells for the first six months of production allows for calibration of early flowback time points and has been shown to provide enough subsurface signal to determine relative variation in out-of-formation contributions.
Understanding temporal variation of a well’s out-of-formation contributions along its lifecycle is a valuable input during field development. Ongoing work with subsurface DNA suggests that out-of-formation contributions vary immediately after wells are bought online and show various trends during production, analogous to many findings supported by independent work in time-lapse geochemistry in the Permian and Bakken.5,6
As a result, when initially profiling a field with the new subsurface diagnostic tool, a well-designed sampling campaign will include wells along all stages of their lifecycles, including parent and legacy wells, to help establish a robust formation-specific signal. Sampling along a time course will provide additional analytical power to calibrate subsurface DNA findings to field geology, operational parameters, and production.

 石油圈
石油圈