Research scientists at King Abdullah University of Science and Technology (KAUST) have developed new forms of metal-organic frameworks (MOFs) that offer substantial potential for use in the oil and gas industry.
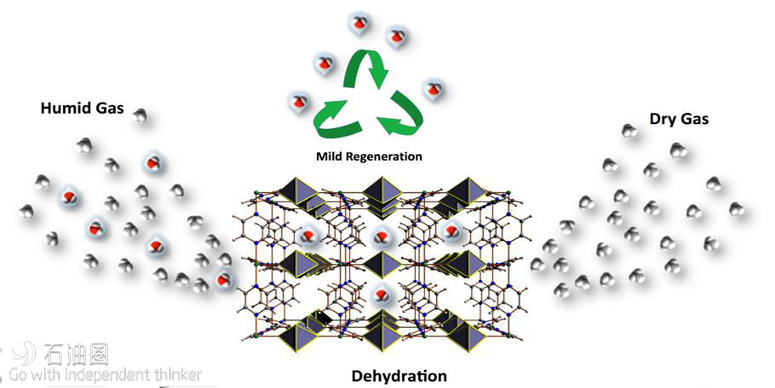
The most recent advancement undercuts the conventional view that MOFs cannot be stable in the presence of water. Professor Mohamed Eddaoudi, director of the Advanced Membranes and Porous Materials Center at KAUST, and his team have recently developed a MOF material that can selectively and effectively absorb water in dry streams.
“The achievement of energy-efficient dehydration by our introduced MOF is revolutionary,” Eddaoudi said.
Gases such as natural gas must be dehydrated before transportation, and dehydrating agents are used to dry the gas and preclude pipeline corrosion and blockages due to methane ice formation. Conventional drying agents require an energy-intensive regeneration cycle.
The new fluorinated MOF achieves the drying and regeneration cycle at relatively low temperatures and requires about half the energy input of conventional procedures. This reduction in energy use highlights the potential for upscaling the innovation to bring huge efficiency gains to the gas production and the transport industries.
MOF removes CO2 and water
MOFs are hybrid organic-inorganic materials that contain metal ions or clusters held in place by organic molecules known as linkers. Varying the metal components and organic linkers allows researchers to fine-tune the structure and chemical properties of MOFs. A major aim of this fine-tuning is to create MOFs with pore systems (cavities and/or channels) that will selectively bind to and retain specific molecules such as the water that must be removed from a gas stream.
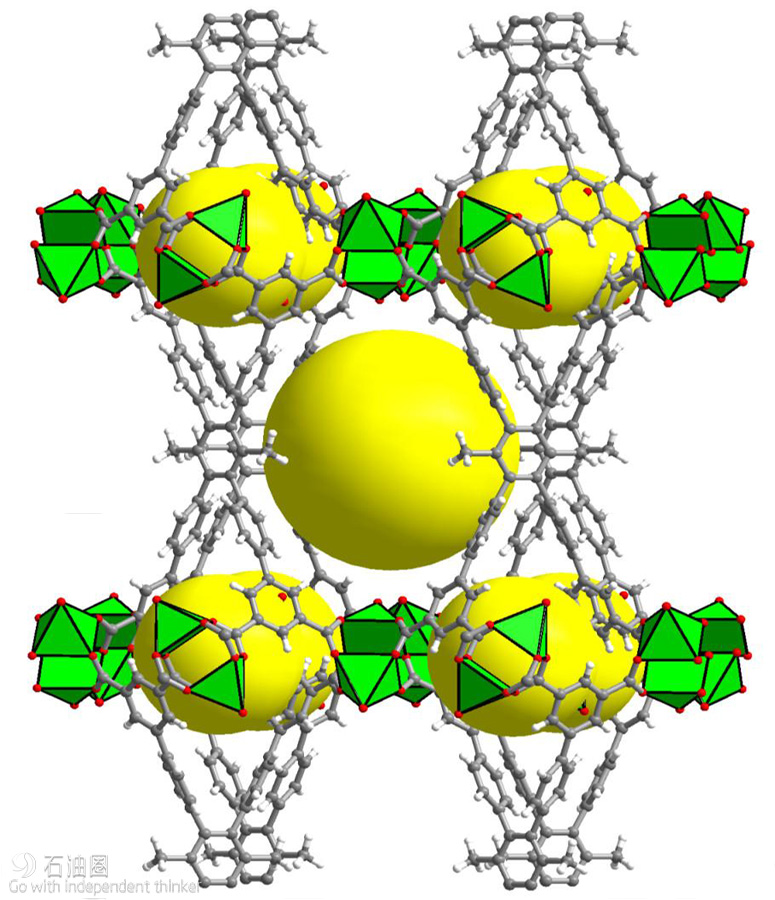
“Initially, our aim was to adapt our recently introduced fluorine-containing MOFs to include a periodic array of open metal sites and fluorine centers in the contracted pore system to achieve various key separations,” Eddaoudi said. This exploration led to the discovery of the water-stable MOF labeled KAUST-8 (Figure 1), containing unique water adsorption properties and recyclable dehydration capabilities. Significantly, KAUST- 8 removes CO2 along with water, which is a common requirement in industrial gas processing.
“I have no doubt that this discovery will inspire scientists in academia and the industry to explore MOFs to address other challenges,” Eddaoudi said. The KAUST team sees additional possibilities, including the removal of water from liquids such as inks and solvents used in the electronics industry.
This is the latest of a string of important advancements that Eddaoudi and his group have unveiled that are ready for deployment in the oil and gas industry. Late last year the research group developed various new MOFs for the effective and efficient storage of gases. They created a hybrid material that could lead to cheaper and more effective methane storage. Since natural gas is almost 95% methane, it is a good candidate for replacing gasoline and coal. Methane is more environmentally friendly in several ways, but its widespread adoption for powering vehicles and other local and mobile applications is limited by shortcomings of existing storage and transport technologies.
“MOFs are considered by far the best class of materials for storing gases, especially methane,” Eddaoudi said. He explained that tinkering with different pore sizes can create exceptionally large internal surface areas that allow MOFs to hold greater amounts of gas than other porous substances. Making MOFs can be likened to using building blocks (Lego chemistry) to assemble a wide range of open geometric networks. Diagrams representing these periodic structures look like colorful toys (Figure 2), only built at the atomic and molecular scale. The key to the latest MOF is the choice of the appropriate organic “pillars” (shown in gray in Figure 2) used to create two types of cavities that can each contain many of the methane molecules taken up by the MOF.
Further tweaking required
“We need to boost the methane storage capacity and cyclability of the MOFs, enhance their stability in water and explore scaling up to commercially useful quantities,” Eddaoudi said.
He predicts that commercially produced MOFs will be efficiently and effectively storing and transporting methane within the next decade. Such a development could herald real progress in weaning society off its dependence on oil and coal.
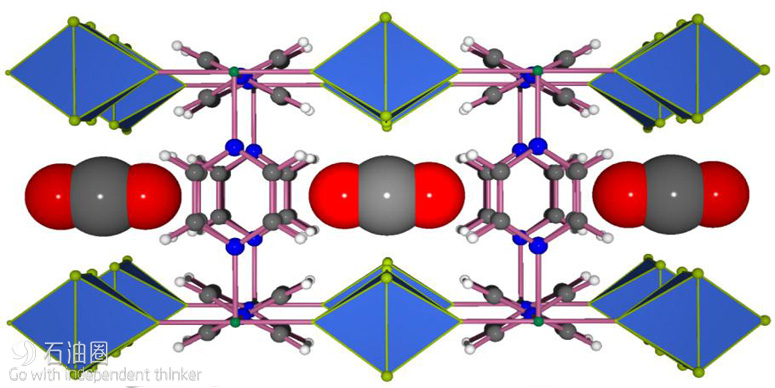
In late 2015 Eddaoudi’s team developed a MOF that can effectively take up CO2 even when it is present at concentrations as low as 400 parts per million and opens possibilities for capturing CO2 as it is generated. The researchers developed a fluorine-containing MOF in which square-grid layers encompassing Ni(II) metal centers and pyrazine linkers are bridged via pillars composed of niobium, oxygen and the fluorine atoms.
“The discovery of this latest material, KAUST-7 [Figure 3], for capturing carbon dioxide is the result of about four to five years of work on this unique MOF platform,” Eddaoudi said. He explained that the key challenge was to create something that could exceed the performance of existing options while also greatly reducing the energy requirements over the full cycle of operation.
Capturing CO2
“The ability to control the distance between the fluorine atoms allowed us to create the ideal squareshaped pockets for trapping carbon dioxide molecules effectively and efficiently and giving our material such impressive performance,” Eddaoudi said.
The ability to trap CO2 when it is at very low concentrations makes the new material suitable for a wide range of applications, including the direct capture from air.
Eddaoudi explained that the MOF (KAUST-7) might be adapted for use in static industrial processes that generate CO2 (such as cement factories) but could also be used onboard vehicles such as trucks, cars and aircraft. Capturing the CO2 as soon as it is emitted could be significantly more cost-effective and energy-efficient than when it has mixed in with the atmosphere overall.

 石油圈
石油圈