Scale formation plagues the oil and gas world. It can be caused by the combination of several different parameters such as brine waters, dissolved solids, pressure and temperature. These components can form a precipitate, especially in situations with incompatible waters. These precipitates coat, block and otherwise cause problems throughout the extraction and refinery processes.
It is possible for a well to go from great production to zero in one day. A classic example took place in the Miller field of the North Sea, where production was near 30,000 barrels per day and dropped to zero in 24 hours. This reduction was caused by scale from mixed waters.
Physical processes, such as drilling (milling), jetting and chemical dissolution, can remove scale once it is formed, but these are reactive and can be costly. In some cases, physical removal does not work as expected and results in a preventable system replacement. In fact, workers sometimes see that after a system has undergone a physical cleanout, the scaling tendency increases. One reason is that the surfaces tend to be rougher than before the physical process. This roughness creates more locations for scale to stick and grow.
The projected cost for preventive chemical treatment over the life of a well is about the same as a single cleanout and reassembly. The major difference in cost between the approaches is the amount of downtime in production, with a cleanout often having significant outage time. Producers also should consider the slowing of production caused by reduced flow. When this lost revenue is accounted for, a proactive stance is much more favorable.
Preventing scaling downhole allows for flow of petroleum fluids to maximize the return on each well. Calcium and barium are commonly found cations, and carbonate and sulfate are common anions (see Table 1).
Many wells have multiple scales, such as a calcium carbonate and calcium sulfate. In this case, a calcium-tolerant inhibitor is a better solution than two different scale inhibitors designed to treat each of the scale types. The thermodynamics and kinetics of scale formation are well understood, but organizations continue to investigate them to find the best method to treat these costly scales.
Long-Term Scale Prevention
Scale prevention is sometimes thought of as simply stopping scale from forming. However, it is not that simple. Oil and gas travel from the ground to the refinery, with scale prevention needed at every point in the process. Two major mechanisms are commonly accepted.
The first is threshold inhibition. This mechanism disrupts the scale cluster before it reaches the critical size needed for nucleation. Nucleation, the physical process of state change (in this case from liquid to solid), must take place before crystallization and scale growth. Rough surfaces increase the number of locations where nucleation can occur, which is why a physical cleanout can lead to a faster scaling of a well.
Scale inhibitors, such as phosphonates and low molecular weight polymers, that act by this mechanism will fail the moment they are overwhelmed. Phosphonates can interact strongly with the scale-forming ions. This is why they are considered good chelating additives. This reaction is desirable, but if the inhibitor starts to get incorporated into a growing crystal lattice, it will begin to be depleted. Although phosphonates can be effective in scale prevention, they have limitations that are especially evident in applications with high temperatures, when degradation of phosphonate occurs.
Additionally, other components such as iron are known to poison threshold inhibitors, creating catastrophic failure that is caused by the chelation of the inhibitor with iron. The affinity toward iron is higher than for calcium, barium or strontium in a system.
The second mechanism for scale prevention is crystal growth blocking. Scale inhibitors that use this mechanism often also use threshold inhibition. But if a crystal does form, the inhibitor can still act to prevent scaling failure. They prevent growth on the face of the crystal, which causes the crystal to distort. The distortion prevents further adhesion, and the crystal remains relatively small.
Polymer Chemistries
Phosphonates are not large enough to cause a distortion, mostly because they have less molecular weight than the crystal. Imagine trying to fill a pothole with a single shovel of stone shale. The pothole will be hit by car tires and increase in size because the shale isn’t enough to fill the pothole. If you fill the same hole with cement, you distort the hole and solve the issue. In terms of scale crystal distortion, phosphonates are like the shale and polymer chemistries are like cement.
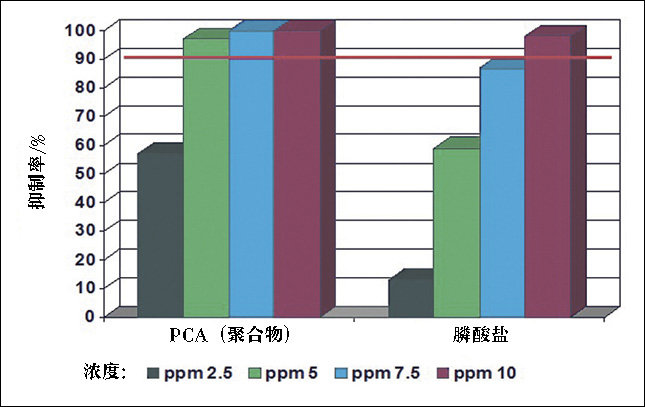
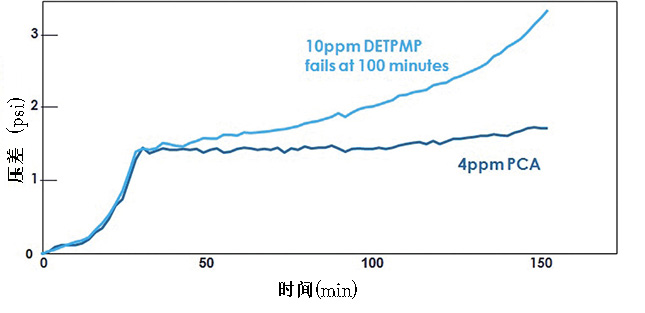
Polymer chemistries, such as those derived from carboxylic and maleic acids, tend to be great for using both scale prevention mechanisms. They are much more thermodynamically stable with greater tolerance to iron. The polymer-based chemistries also have greater compatibility with other commonly used additives than phosphonates do. Figure 1 shows how polymers and phosphonates can compare for a threshold test. Figure 2 demonstrates polymers’ superior performance in a tube blocking test.
Polymer chemistries also have a broader spectrum capability. For example, polycarboxylic acid (PCA) polymers can be excellent inhibitors against calcite. However, scales rarely occur singularly so barite might also be in the system. The broader abilities of the PCA could also handle the barite if it occurred in small enough amounts.
Choosing a System
If a user has a short-term focus, scale formation will probably occur and cause problems from the wellbore to the fittings, couplings and piping system on the surface. In fact, the surface area, where the temperatures and pressures have reduced, needs as much protection as the well. The change in conditions from down well to topside can adversely affect the scaling risk.
Preventive measures result in an overall lower cost for a producer and deliver a more reliable, predictable system. Considering everything that can go wrong, mitigating scale formation by using a quality polymer chemistry is an effective approach.

 石油圈
石油圈