The use of nanomaterials for alteration of wettability is a method that has grown in prominence after the development of techniques for synthesizing nanosized particles in the late 1980s. In this paper, after a review of the fundamentals of wettability alteration, a discussion of nanomaterials used for wettability alteration is provided. Among these nanomaterials, nanoparticles of silica and polysilicone indicate better results in terms of efficiency on incremental oil recovery in waterflooding.
Introduction
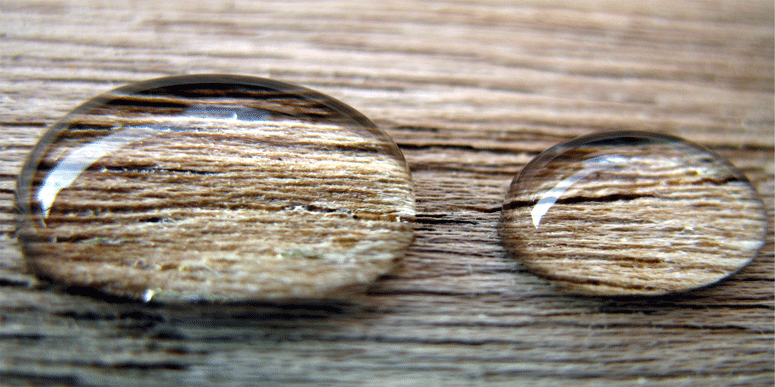
Wettability is the tendency of a fluid to spread over a specific surface and is relative to other existent fluids in that system and is defined by the contact angle of a droplet of the fluid and the surface. It is a result of adhesion forces between the fluid and the minerals of the rock. The wettability of a rock ranges from strongly water-wet to strongly oil-wet and is a result of brine/oil/rock interactions in a reservoir. There are different types of rocks on the basis of these interactions and wettabilities:
1.If no, or equal, tendency is shown from oil or brine to spread over the surface of the rock, the system is said to have neutral wettability or intermediate wettability.
2.Because different mineralogies coexist in an oil reservoir, different wettabilities are also expected. If this variety in the reservoir is not negligible, then, in different parts of the reservoir, different chemical interactions between fluids and rocks are observed and, consequently, some areas of the reservoir indicate strongly water-wet behavior whereas some other areas indicate strongly oil-wet behavior. This heterogeneous wettability behavior is known as fractional wettability.
3.In some cases, the smaller pores are occupied by water and can be considered water-wet, while larger pores are captured by oil. This type of wettability distribution is known as mixed wettability, in which the residual oil saturation is low because the oil is displaced more easily from larger pores.
The solid/fluid and fluid/fluid surface energies are governed by the chemical compositions of the fluid and rock. In other words, the mineralogy of a rock and chemical properties of the fluid influence the relative adhesive tensions and, consequently, wettability.
The most common methods for wettability measurements, discussed in detail in the complete paper, include the following:
1.Amott wettability index
2.US Bureau of Mines (USBM) wettability index
3.Combined Amott-USBM wettability test
4.Contact-angle methods
Because any clean rock exhibits water-wetting behavior, it is believed that all petroleum reservoirs were initially water-wet. This water was later displaced by oil because of migration, and sometimes there is a shift to relatively oil-wet compared with the initial wetting tendency. Some polar components of oil then act as surfactants and penetrate through the thin film of water on the pore surfaces and adsorb strongly on the rock.
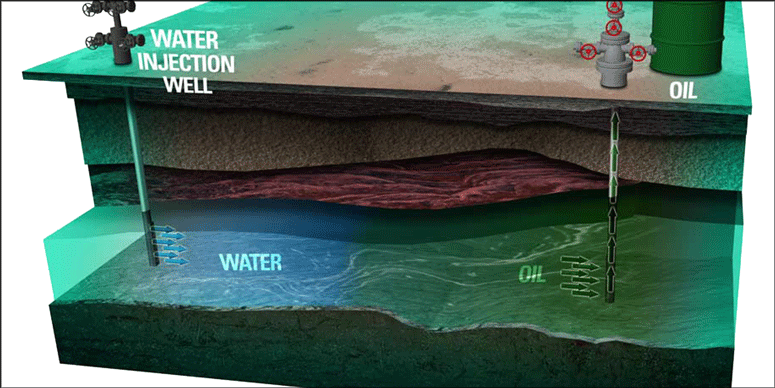
On the basis of the component minerals, some rocks have water-wetting or oilwetting natures. In a water-wet medium, water captures the small pores and also coats the surface of the larger pores while oil filaments are in the larger pores on the mentioned water surface. The water relative permeability in larger pores is small both before coreflooding (because presence of oil prohibits water mobility) and after coreflooding (because the residual oil saturation impedes water relative permeability).
In an oil-wet system, the positions of the fluids are reversed, and during waterflooding, the water relative permeability in larger pores increases and impedes the oil movement faster than in a water-wet system. In other words, an oil-wet system is not a good candidate for waterflooding compared with a water-wet system because more oil would reside in an oil-wet system after water breakthrough. In some cases in which the oil-/water-viscosity ratios are high, breakthrough happens very early and the residual oil saturation becomes significant.
Wettability-Alteration Methods
Several methods are used to alter wettability. Most of these methods cannot be used in large scale because of their expense and are only used to treat small cores for experiments that require cores of different wettabilities. These wettability-alteration methods include the following.
Treatment With Organosilanes. Organo-silanes are widely used as wettability-altering agents in different industries. In other fields, they are used as hydrophobic agents, which can be interpreted as oil-wetting agents in the petroleum industry.
Treatment With Naphthenic Acids.
Naphthenic acids are long chains of heavy organic compounds; the label refers to all the carboxylic acids seen in crude oil. They are viscous and insoluble in water but completely soluble in organic solvents and oil. Because of their toxicity, environmental hazards, and high viscosity, they can be used only in laboratory scales to alter wettability. In general, naphthenic acids make a carbonate core more oil-wet because of the reaction of the naphthenic acid and calcium carbonates. However, silica cores show the opposite behavior.
Treatment With Asphaltenes. Asphaltenes are heavy componenets of crude oil that are considered polar. As mentioned previously, all rocks are considered to be initially water-wet, although during oil migration some of them turned partially oil-wet. Presence of asphaltenes in crude oil is one of the reasons for this wettability alteration. Asphaltenes rupture the thin film of water and are ad-sorped to the rock surface in large pores, which can cause the larger pores to exhibit oil-wetting behavior.
Thermal Methods. During the oil migration that turned water-wet rocks to partially oil-wet rocks, when a critical capillary pressure was reached, the heavy oil components penetrated through the thick water films on the pore surfaces and, by deposition on the surface, made the surface oil-wet. This process can be reversed by heating in silicate rocks. Heating causes the deposited (adsorbed) active agents to be desorbed, leaving a water-wet surface again. Most of the naturally fractured reservoirs show oil-wetting behavior, and, therefore, waterflooding would not be successful in these systems. Water will imbibe into the cores but will only pass through the fractures and result in very low recoveries. However, by heating the reservoir by hot water or steam injection, the system could exhibit more-water-wetting behavior and higher recoveries may result. The water front locates the wetting transition.
Surfactants. Surfactants are the only materials also used on a large scale to alter the wettability and improve the recovery. By use of these surfactants and caustics, the interfacial tension is reduced and more oil is produced; however, because of adsorption of these surfactants and the precipitation caused by the presence of divalent cations in the brine, only 5% incremental oil is produced. Because of the high residual oil saturation, an oil-wet reservoir is typically not a good candidate for waterflooding; however, by use of surfactants, the interfacial tension can be reduced or the wettability can be shifted toward a more-water-wetting system, which improves the recovery factor.
Wettability Alteration With Nanomaterials
Use of nanomaterials is a recent, and in many cases highly effective, method of wettability alteration. Because the sizes of particles are reduced, their effectiveness is greatly increased. However, this precision in wettability alteration causes the method to become more expensive and less feasible.
In most cases, the wettability of a core has to be modified to reach a preferable oil recovery; however, in some rare cases (mostly experimental), a surface with a specific wettability is needed and therefore the fabrication of surfaces with a specific wettability is required. Adjusting the wettability of a surface by nanostructures while it is being fabricated can be achieved by electrochemical methods, plasma etching, or electrospraying.
Nanopolysilicone (NPS) is a nanoparticle used to alter the wettability in cores. NPSs are classified into three groups: lyophobic and hydrophilic polysilicone (LHP), neutrally wetting polysilicone (NWP), and hydrophobic lipophilic polysilicone (HLP).
By adsorption of LHP on the pore surface, the oil-wetting system would be altered to water-wet, which would increase the relative permeability of oil and decrease the oil relative permeability. This situation is favorable in a waterflooding process. NWP adsorption reduces the surface tension and increases the oil relative permeability generally, which is also favorable. However, adsorption of HLP is not favorable because it makes the pore surface oil-wet, which would increase the residual oil saturation in a flooding process. HLPs are transferred by diffusion and convection, and, in the case of accumulation, they would cause pore blockage and reduction in porosity and absolute permeability.
HLP makes a system more oil-wet, and, in contrast, LHP makes a system more water-wet. By adding LHP to a water-wet system, the media become extremely water-wet, which would hinder fluid movement in flooding. The third type of NPS, which is NWP, reduces the interfacial tension between the oil and formation water. Each of these nanoparticles needs a carrier fluid that preserves particle wetting characteristics. Alcohols are suggested for use with NWP and HLP; in particular, ethanol is reported to be the best choice because it is converted from a very weak surfactant to a very good surfactant and reduces the interfacial tension significantly. Water is suggested to be used as a carrier fluid while using LHP.
As mentioned previously, an oil-wet system results in a high residual oil saturation after flooding, while a water-wet system is typically a better candidate for waterflooding. However, the best system for flooding is an intermediate system in which, ideally, no preference for any of the fluids is shown from the rock surface. Therefore, the aim of any wettability-alteration process would be to alter the system to an intermediate status.

 石油圈
石油圈